
Introduction:
In biological research, the fusion of biology, computer science, and statistics has given birth to an exciting field called bioinformatics. The advent of bioinformatics has revolutionized biological research by providing computational tools and techniques to analyze and interpret complex data. With its ability to analyze and interpret vast amounts of complex biological data, bioinformatics has emerged as a critical discipline in the “Age of Bioinformatics.” This comprehensive article explores the pivotal role of bioinformatics in advancing biological research, focusing on its real-life applications, remarkable achievements, and the machine learning tools that have propelled the field forward.
Bioinformatics: A Haven for Data Scientists and Machine Learning Engineers:
Bioinformatics offers an unparalleled opportunity for data scientists and machine learning engineers to apply their expertise in solving complex biological problems. The field demands a unique combination of computational skills and biological knowledge, making it a perfect match for individuals with a data science and machine learning background. By leveraging their data analysis, pattern recognition, and predictive modeling skills, they can unearth valuable insights from massive biological datasets, enabling breakthrough discoveries and accelerating scientific progress.
Real-Life Applications and Triumphs of Bioinformatics:
In several instances, bioinformatics has triumphed over traditional methods, showcasing its immense potential. For example, in genomics, the Human Genome Project utilized bioinformatics tools and techniques to sequence and analyze the human genome, revolutionizing our understanding of genetic diseases. Bioinformatics has also played a crucial role in comparative genomics, where researchers compare the genomes of different species to identify evolutionary relationships and gain insights into the genetic basis of diseases.
In proteomics, bioinformatics tools have been instrumental in deciphering the complex world of proteins. By combining data from mass spectrometry experiments and sequence databases, researchers can identify and characterize proteins, understand their functions, and explore their interactions with other molecules. These insights have contributed to advancements in fields like drug discovery, where bioinformatics has aided in identifying potential drug targets, designing novel molecules, and predicting drug efficacy, significantly reducing time and cost in the development process. Some applied sub-fields focused on exploring how bioinformatics has enabled the sequencing and analysis of genomes, leading to breakthroughs in personalized medicine, genetic diseases, and evolutionary biology.
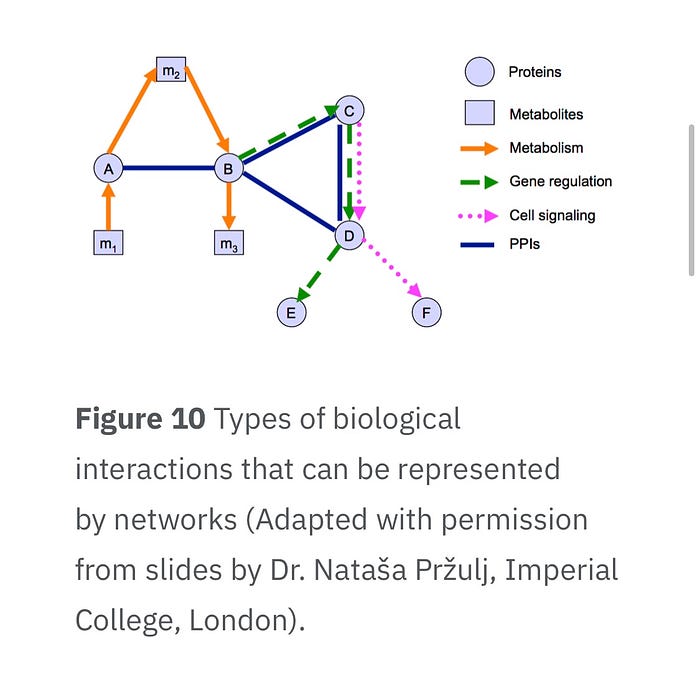
Proteomics and Bioinformatics: Proteomics, the study of proteins and their functions, relies heavily on bioinformatics for data analysis and interpretation. This section explores how bioinformatics tools have facilitated protein identification, characterization, and interaction analysis, contributing to advancements in drug discovery and understanding complex biological processes.
Transcriptomics and Bioinformatics: Transcriptomics, the study of gene expression patterns, has benefited from bioinformatics approaches. This section delves into the use of bioinformatics tools for analyzing gene expression data, identifying differentially expressed genes, and uncovering regulatory networks.
Metagenomics and Bioinformatics: Metagenomics, the study of genetic material recovered directly from environmental samples, presents unique computational challenges. This section highlights how bioinformatics enables the analysis of metagenomic data, helping researchers understand microbial communities and their impact on ecosystems and human health.
Genomics and Bioinformatics: Genomics, the study of an organism’s complete set of DNA, has been dramatically influenced by bioinformatics. This section discusses how bioinformatics has enabled the sequencing and analysis of genomes, leading to breakthroughs in genetic diseases and evolutionary biology.
Advances in Bioinformatics
The field of bioinformatics has witnessed remarkable advancements in recent years. One key factor driving progress is the development of high-throughput technologies. Next-generation sequencing (NGS) platforms have dramatically increased the speed and reduced the cost of DNA sequencing, leading to the generation of vast amounts of genomic data. Bioinformatics algorithms and tools have played a crucial role in analyzing NGS data, enabling researchers to study genetic variations, gene expression patterns, and epigenetic modifications on a large scale.
Advancements in mass spectrometry have revolutionized proteomics, allowing for the identification and quantification of thousands of proteins simultaneously. Bioinformatics tools have been developed to process and interpret complex datasets, facilitating understanding of protein functions, interactions, and post-translational modifications. Another area of advancement in bioinformatics is the integration of multi-omics data. Researchers can comprehensively understand biological systems by combining information from genomics, transcriptomics, proteomics, and other omics fields. This integration requires sophisticated computational methods, such as data integration algorithms and network analysis approaches, which enable extracting meaningful insights from multiple layers of biological data.

Challenges in the Field of Bioinformatics
Despite its tremendous advancements, Bioinformatics faces several challenges that must be addressed to harness its potential fully. The following are some critical challenges in the field:
a) Data Integration: With the advent of high-throughput technologies, enormous volumes of biological data are being generated from diverse sources. Integrating and analyzing data from multiple platforms and experiments pose challenges due to data formats, normalization techniques, and data quality differences. Developing robust data integration and harmonization methods is essential to derive meaningful insights from heterogeneous datasets.
b) Data Privacy and Security: As bioinformatics deals with sensitive genetic and health-related information, ensuring data privacy and security is crucial. Anonymizing and protecting individual identities while maintaining data utility is a significant challenge. Robust data protection measures, secure data-sharing platforms, and adherence to ethical guidelines and regulations are necessary to safeguard personal information.
c) Algorithm Robustness and Validation: Bioinformatics algorithms and tools must be rigorously validated to ensure their accuracy, reproducibility, and generalizability across different datasets and biological contexts. Developing benchmark datasets and standardized evaluation metrics is necessary to assess algorithm performance and facilitate comparisons between other methods.
d) Interpretability and Explainability: With the increasing complexity of bioinformatics models, the interpretability and explainability of their predictions have become crucial. Understanding the underlying biological mechanisms and features driving model predictions is essential for gaining trust in the results and facilitating their application to real-life scenarios. Developing methods for model interpretability and explainability is an active area of research in bioinformatics.
e) Big Data Analytics: The exponential growth of biological data presents challenges in storing, processing, and analyzing large-scale datasets. Traditional computational infrastructure may not be sufficient to handle the vast amounts of data generated by high-throughput technologies. Developing scalable and efficient algorithms and leveraging cloud computing and parallel processing techniques are necessary to tackle significant data challenges in bioinformatics.
f) Standardization and Reproducibility: Ensuring standardization and reproducibility of bioinformatics analyses is vital for scientific integrity. A lack of standardized protocols, software versions, and data repositories can hinder the reproducibility of results across different research groups. Developing community-driven standards, guidelines, and open-source tools can address these challenges and promote transparency and collaboration in the field.
g) Training and Education: Bioinformatics requires a multidisciplinary skill set encompassing biology, computer science, and statistics. However, the rapid pace of technological advancements and the evolving nature of the field pose challenges in providing adequate training and education opportunities. Bridging the gap between biology and computational disciplines through interdisciplinary training programs can address this challenge.
h) Ethical Considerations: Bioinformatics raises ethical considerations related to data privacy, informed consent, and potential misuse of genetic information. Balancing the benefits of data sharing and open science with individual privacy rights and ethical principles is essential. Developing ethical frameworks, guidelines, and regulations to govern the responsible use of bioinformatics data and technologies is critical.
While bioinformatics has made remarkable strides in advancing biological research, it faces several challenges that must be overcome. Addressing the challenges of data integration, privacy and security, algorithm robustness and validation, interpretability and explainability, big data analytics, standardization, and reproducibility, training and education, and ethical considerations will pave the way for further advancements in the field. By collaboratively addressing these challenges, bioinformatics can continue to drive transformative discoveries and improve our understanding of complex biological systems for the betterment of human health and beyond.
Future Perspectives and Opportunities
As the Age of Bioinformatics progresses, numerous exciting opportunities are on the horizon. Integrating multi-omics data will continue to be a significant focus, as it promises a comprehensive understanding of biological systems. The development of explainable and interpretable machine learning models will enhance the trustworthiness of predictions and enable researchers to gain deeper insights into the underlying biological mechanisms.
Additionally, the fusion of bioinformatics with other emerging technologies, such as artificial intelligence, robotics, and quantum computing, holds tremendous potential for further advancements in biological research. For example, applying artificial intelligence algorithms, such as reinforcement learning, can optimize experimental design and guide the discovery of new drugs or biomarkers. Robotics and automation can streamline laboratory workflows, enabling high-throughput experimentation and data generation. Quantum computing may offer computational advantages for solving complex biological problems, such as protein folding prediction or large-scale molecular simulations.
Machine Learning Tools in Bioinformatics
Machine learning is vital in bioinformatics, providing data scientists and machine learning engineers with powerful tools to extract knowledge from biological data. Supervised learning algorithms, such as support vector machines and random forests, have been extensively used in bioinformatics for tasks like classifying biological samples and predicting outcomes. For example, these algorithms have been applied to predict disease outcomes based on genomic data or to classify cancer subtypes based on gene expression profiles.
Unsupervised learning techniques, such as clustering and dimensionality reduction, aid in identifying patterns and structures within datasets. Clustering algorithms can group similar biological samples or identify distinct subtypes within a disease. Dimensionality reduction methods, such as principal component analysis and t-distributed stochastic neighbor embedding, help visualize high-dimensional data and identify the most informative features.
Deep learning, a subset of machine learning, has revolutionized image analysis in bioinformatics. Convolutional neural networks (CNNs) have been successfully applied to tasks such as image-based cell classification, protein structure prediction, and drug discovery. By learning from large-scale image datasets, these models can automatically extract features and patterns, enabling faster and more accurate analysis of biological images.
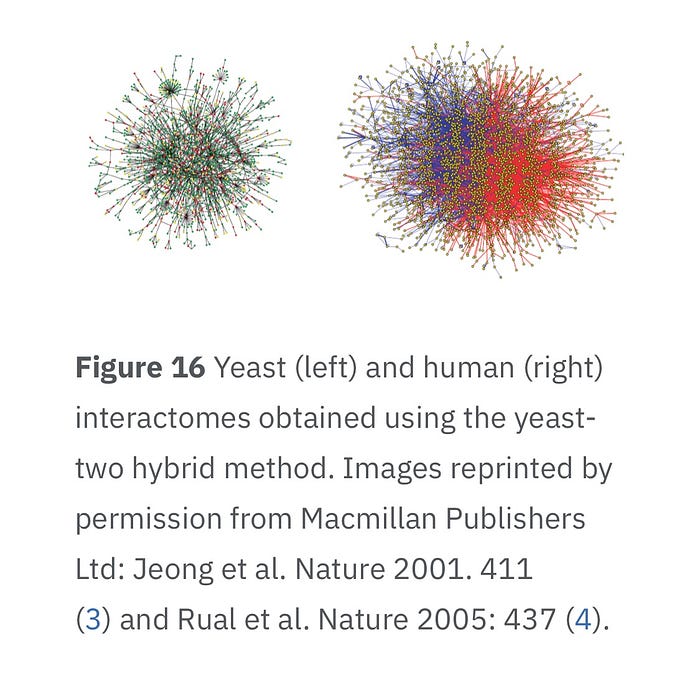
Conclusion
The Age of Bioinformatics has transformed the landscape of biological research, empowering data scientists and machine learning engineers with computational tools and approaches to unravel the complexities of biological systems. Bioinformatics has proven its worth in revolutionizing biological research through its real-life applications and remarkable achievements. With continual advancements in algorithms and machine learning tools, the field is poised to make even more significant strides in the future.
By embracing the collaborative potential between bioinformatics and data science, we can expect to witness further breakthroughs that will transform our understanding of life and revolutionize the practice of medicine, emphasizing the role of machine learning in bioinformatics and showcasing real-life applications that have revolutionized disease understanding, drug discovery, and precision medicine. As bioinformatics evolves and addresses challenges and ethical considerations, it promises further advancements and applications in various fields.